
Peptide Chemistry
Palladium-Catalyzed C(sp3)–H Arylation of Peptides Assisted by Unmodified Asparagine
Contributors: Hisashi Yamamoto, Tomohiro Hattori
Weng Y, Ding X, Oliveira JC. A, Xu X, Kaplaneris N, Zhu M, Chen H, Chen Z, Ackermann L.
Zhejiang University of Technology, Hangzhou, P. R. of China and Georg-August-Universität Göttingen, Germany
Chem. Sci. 2020;
11: 9290-9295
DOI: 10.1039/d0sc.03830j.
Key words
palladium catalysis - C–H activation - arylation - asparagine
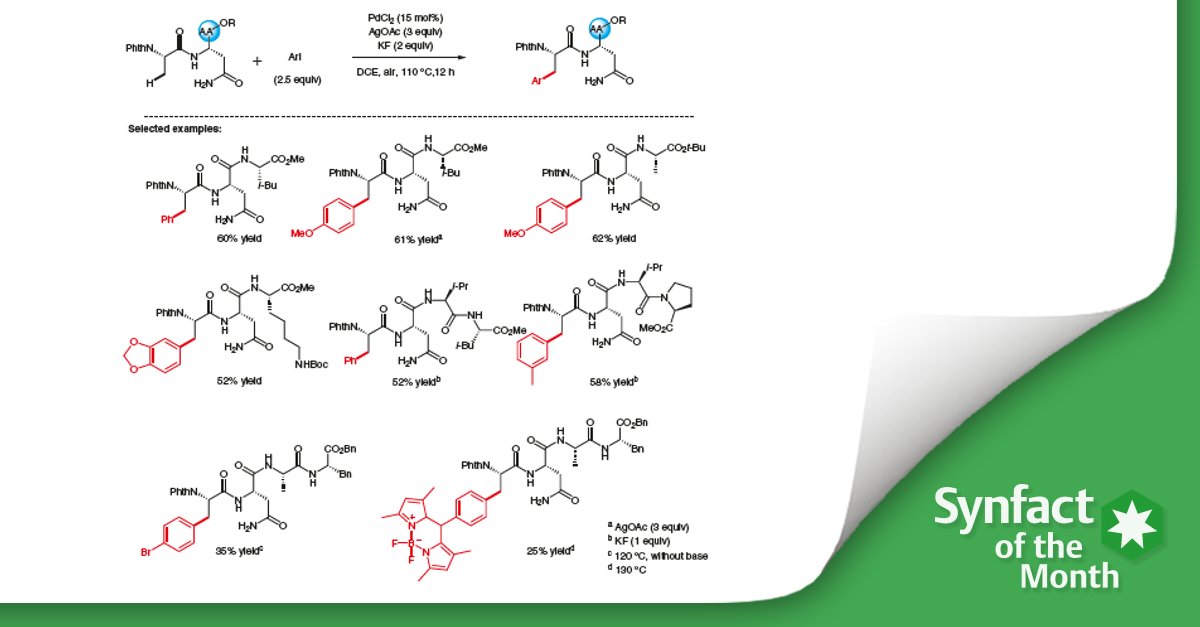
Significance
Late-stage modification of peptides has emerged as an invaluable method in synthetic chemistry. The authors report a C(sp3)–H arylation of peptides by a palladium-catalyzed reaction with internal asparagine (Asn) as a directing group.
Comment
The site-selective C(sp3)–H arylation proceeded smoothly at the N-termini of di-, tri-, or tetrapeptides, assisted by the unmodified side chain of Asn, without any exogenous directing group.
