
Thieme Chemistry
Keep Track of the Latest Content
我们很高兴地与您分享本月Thieme Chemistry最新一期的精彩内容:
SYNTHESIS Special Issue:
Bond Activation – in Honor of Prof. Shinji Murai: Part I & Part II
Editor: Hideki Yorimitsu教授
Guest editor: Naoto Chatani教授
SYNLETT Cluster:
Perspectives on Organoheteroatom and Organometallic Chemistry
Editor: 李昂教授
Guest editor: 姜雪峰教授
Organic Materials Focus Issue:
Supramolecular Optoelectronic Materials
Editors: Subi J. George, Pol Besenius
欢迎免费阅读。
Synthesis
Bond Activation – in Honor of Prof. Shinji Murai
Part I + II
Korkit Korvorapun, Ramesh C. Samanta, Torben Rogge, Lutz Ackermann
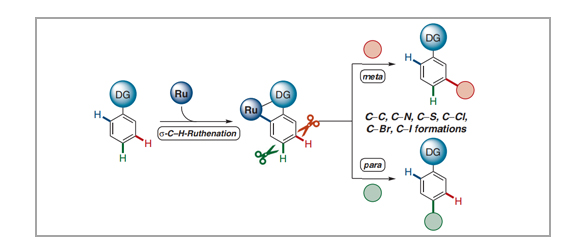
Synthetic transformations of otherwise inert C–H bonds have emerged as a powerful tool for molecular modifications during the last decades, with broad applications towards pharmaceuticals, material sciences, and crop protection. Consistently, a key challenge in C–H activation chemistry is the full control of site-selectivity. In addition to substrate control through steric hindrance or kinetic acidity of C–H bonds, one important approach for the site-selective C–H transformation of arenes is the use of chelation-assistance through directing groups, therefore leading to proximity-induced ortho-C–H metalation. In contrast, more challenging remote C–H activations at the meta- or para-positions continue to be scarce. Within this review, we demonstrate the distinct character of ruthenium catalysis for remote C–H activations until March 2021, highlighting among others late-stage modifications of bio-relevant molecules. Moreover, we discuss important mechanistic insights by experiments and computation, illustrating the key importance of carboxylate-assisted C–H activation with ruthenium(II) complexes.
Recent Advances in Heterogeneous Ir Complex Catalysts for Aromatic C–H Borylation
Kyogo Maeda, Ken Motokura
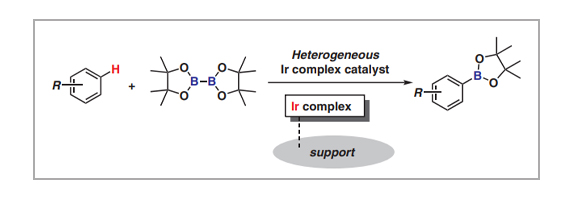
Aromatic C–H borylation catalyzed by an Ir complex is among the most powerful methods for activating inert bonds. The products, i.e., arylboronic acids and their esters, are usable chemicals for the Suzuki–Miyaura cross-coupling reaction, and significant effort has been directed toward the development of homogeneous catalysis chemistry. In this short review, we present a recent overview of current heterogeneous Ir-complex catalyst developments for aromatic C–H borylation. Not only have Ir complexes been immobilized on support surfaces with phosphine and bipyridine ligands, but Ir complexes incorporated within solid materials have also been developed as highly active and reusable heterogeneous Ir catalysts. Their catalytic activities and stabilities strongly depend on their surface structures, including linker length and ligand structure.
Synlett
Perspectives on Organoheteroatom
and Organometallic Chemistry
Editor: 李昂教授
Guest editor: 姜雪峰教授
Deqing Hu, Xuefeng Jiang
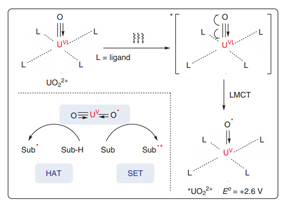
The application of uranyl salts as powerful photoredox catalysts in chemical transformations lags behind the advances achieved in thermocatalysis and structural chemistry. In fact, uranyl cations (UO2 2+) have proven to be ideal photoredox catalysts in visible-light-driven chemical reactions. The excited state of uranyl cations (*UO2 2+) that is generated by visible-light irradiation has a long-lived fluorescence lifetime up to microseconds and high oxidizing ability [E o = +2.6 V vs. standard hydrogen electrode (SHE)]. After ligand-to-metal charge transfer (LMCT), quenching occurs with organic substrates via hydrogen-atom transfer (HAT) or single-electron transfer (SET). Interestingly, the ground state and excited state of uranyl cations (UO2 2+) are chemically inert toward oxygen molecules, preventing undesired transformations from active oxygen species. This review summarizes recent advances in photoredox transformations enabled by uranyl salts.
Organic Materials
Focus Issue:
Supramolecular Optoelectronic Materials
Editors: Subi J. George, Pol Besenius
Solvent-Free Conjugated Polymer Fluids with Optical Functions
Akira Shinohara, Zhenfeng Guo, Chengjun Pan, Takashi Nakanishi
Solvent-free fluidic materials possessing optoelectronic functions are expected to be major components in soft electronics applications. Conjugated polymers are promising targets for this purpose and their design approaches are classified into three types with respect to their structure: conjugation breaking (Type I), copolymerization with flexible polymers (Type II), and side chain engineering (Type III). In this short review, we highlight several early attempts to produce Type III conjugated polymers. We also present fully characterized Type III fluids recently developed by our group, with a brief summary of the structure–property relationship and fluidity-oriented functions.

