Thieme Chemistry
SYNFACTS · SYNLETT · SYNTHESIS
Keep Track of the Latest Content
Our Highlighted Articles and Specials
为了应对复杂结构和生物活性分子合成的当代挑战,自由基反应越来越重要。由苏州大学材料与化学化工学部朱晨教授和南方科技大学化学系刘心元教授为客座主编的SYNLETT Cluster Radicals – by Young Chinese Organic Chemists,将让你了解到该领域已取得的重大进展。
同时,欢迎您免费阅读Thieme化学期刊Synthesis和Synfacts的本月精选论文。
▼

F. Wech et al.
We herein describe the two-step synthesis of 6-adamantyl-2-pyridone from 1-acetyladamantane. The borane complex derived from 6-adamantyl-2-pyridone and the Piers borane liberates dihydrogen at 60 °C. The reverse reaction, hydrogen activation by the formed pyridonate borane is accomplished under mild conditions. The mechanism of the hydrogen activation is studied by DFT computations.

作者:张慧慧、吉梅山、魏猷浩、陈浩东、吴新鑫、 朱晨
A radical-mediated hetaryl functionalization of nonactivated alkenes through distal ipso-migration of O- or S-containing hetaryls was developed. Furyl, benzofuryl, thienyl, and benzothienyl groups showed satisfactory migratory abilities. A variety of heteroatom-centered radicals, including azido, trifluoromethylsulfanyl, and silyl radicals readily trigger the migration cascade, and a new C–heteroatom and C–C bond are concomitantly constructed in the reaction. This method provides an efficient approach to the synthesis of high-valued complex O- or S-hetaryl compounds.

作者:刘丹、 张晶、 陈以昀
The alkoxyl radicals have demonstrated superior hydrogen atom transfer reactivity in organic synthesis due to the strong oxygen–hydrogen bond dissociation energy. However, only the intermolecular hydrogen atom transfer (HAT) and intramolecular 1,5-HAT have been widely studied and synthetically utilized for C(sp3)–H functionalization. This Account summarizes our investigations on the unusual 1,2-HAT reactivity of alkoxyl radicals under visible-light-induced reaction conditions for the α-C–H functionalization. Various mechanistic investigations were discussed in this Account to address three key questions to validate the 1,2-HAT reactivity of alkoxyl radicals.
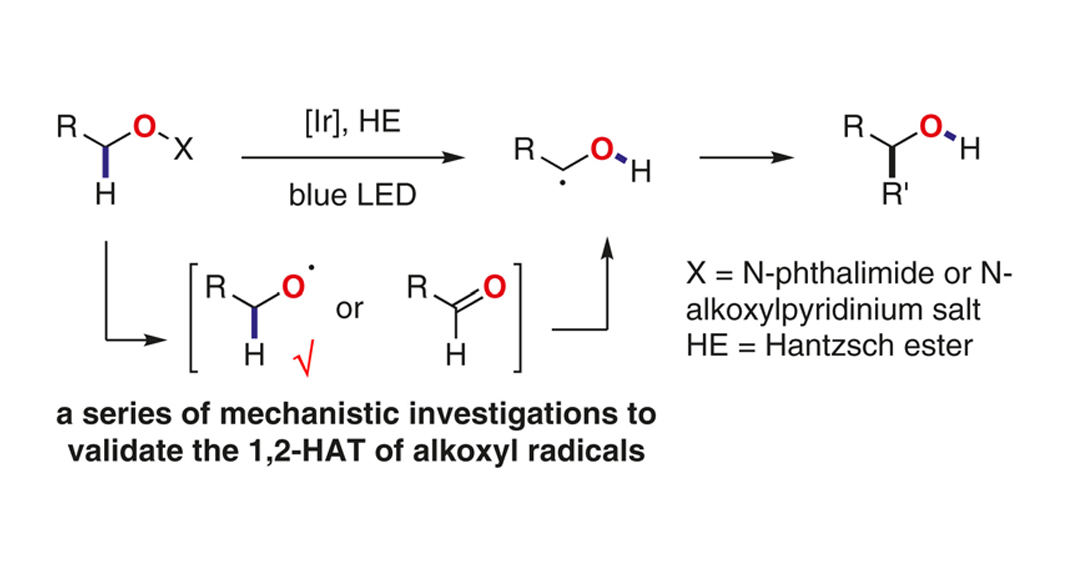
阅读Cluster Radicals – by Young Chinese Organic Chemists的所有论文,请点击这里。

Contributor(s): Paul Knochel, Johannes H. Harenberg
Significance
The authors report a ruthenium-catalyzed hydrogenation of thioesters, thiocarbamates and thioamides. The procedure provides waste-free access to alcohols, thiols and amines. An aciridine-based ruthenium complex is used as catalyst. The products are obtained in excellent yields. Whereas ketones and terminal alkenes are reduced, esters, amides, carboxylic acids and tertiary alkenes are tolerated by the hydrogenation protocol.
Comment
A plausible reaction mechanism based on mechanistic studies is proposed. Interestingly, the aldehyde is hydrogenated when used as a substrate in the presence of the thiol, suggesting an outer-sphere transition state. Other than the temperature, increasing the hydrogen pressure did not improve the yield significantly. It is assumed that heating facilitates the dissociation of the thiol from the ruthenium catalyst.