
SYNTHESIS
Reviews and Full Papers in
Chemical Synthesis
影响因子 2019:2.675

Direct Access to Highly Functionalised Benzimidazoles and Benzoxazoles from a Common Precursor
从一个共同的前体直接获得高度功能化的苯并咪唑和苯并恶唑
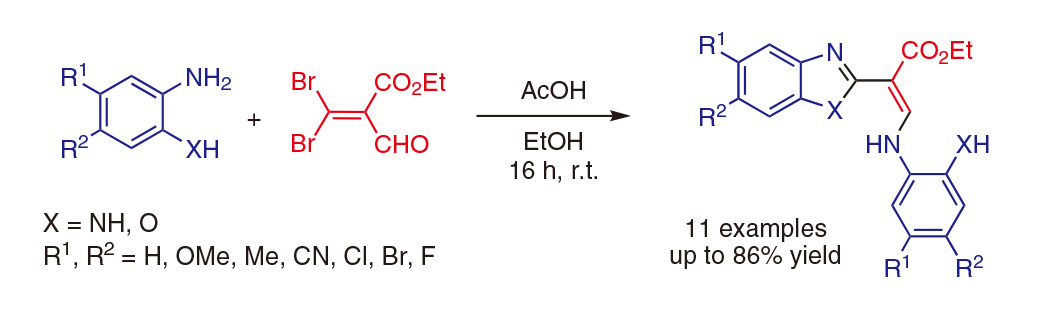
苯并恶唑和苯并咪唑是药物化学中常见的杂环化合物,它们在1-、2-、5-和/或6-位上的功能化使其具有广泛的生物学意义。在这篇文章中,我们直接制备了在这些位置上被高度取代的各种苯并咪唑和苯并恶唑,从一种常见的原料3,3-二溴丙烯醛开始描述。这类丙烯醛衍生物几乎从未在文献中被描述,或者是用作有机合成的“基石”。发现该底物的双重亲电子性有利于与两个当量的各种1,2-二氨基苯或2-氨基苯酚衍生物缩合。在无金属和温和条件下进行的一锅法反应可产生三个新的碳杂原子键,并提供所需的杂环。
Benzoxazole and benzimidazole are commonly encountered heterocycles in medicinal chemistry and their functionalisation around 1-, 2-, 5-, and/or 6-positions provides a wide range of molecules of biological interest. In this manuscript, a straightforward preparation of diversely and highly substituted benzimidazoles and benzoxazoles on these positions, from a common starting material, a 3,3-dibromoacrolein, is described. Such acrolein derivatives are almost never described in the literature or used as ‘building-block’ for organic synthesis. The double electrophilicity of this substrate was found to be advantageous for condensation with two equivalents of various 1,2-diaminobenzene or 2-aminophenol derivatives. This one-pot reaction performed under metal-free and mild conditions allows the creation of three new carbon–heteroatom bonds and affords the desired heterocycles.
