
Pharmacopsychiatry
Impact Factor 2019: 4.340
药理学Pharmacopsychiatry 2021年第1期已经上线,欢迎阅读本期主编精选论文。
Review and Consensus on Pharmacogenomic Testing in Psychiatry
Bousman CA et al.
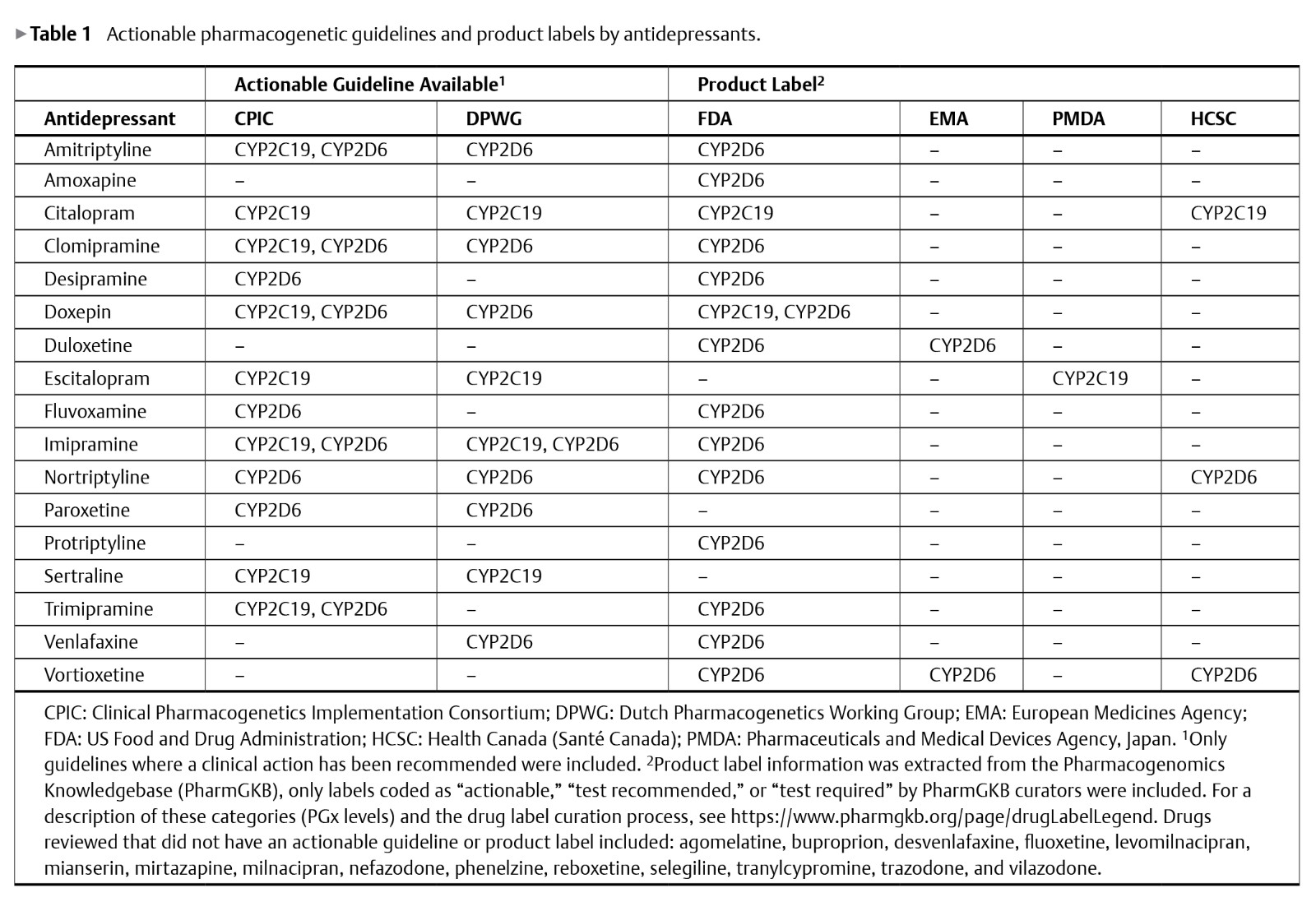
The implementation of pharmacogenomic (PGx) testing in psychiatry remains modest, in part due to divergent perceptions of the quality and completeness of the evidence base and diverse perspectives on the clinical utility of PGx testing among psychiatrists and other healthcare providers. Recognizing the current lack of consensus within the field, the International Society of Psychiatric Genetics assembled a group of experts to conduct a narrative synthesis of the PGx literature, prescribing guidelines, and product labels related to psychotropic medications as well as the key considerations and limitations related to the use of PGx testing in psychiatry. The group concluded that to inform medication selection and dosing of several commonly-used antidepressant and antipsychotic medications, current published evidence, prescribing guidelines, and product labels support the use of PGx testing for 2 cytochrome P450 genes (CYP2D6, CYP2C19). In addition, the evidence supports testing for human leukocyte antigen genes when using the mood stabilizers carbamazepine (HLA-A and HLA-B), oxcarbazepine (HLA-B), and phenytoin (CYP2C9, HLA-B). For valproate, screening for variants in certain genes (POLG, OTC, CSP1) is recommended when a mitochondrial disorder or a urea cycle disorder is suspected. Although barriers to implementing PGx testing remain to be fully resolved, the current trajectory of discovery and innovation in the field suggests these barriers will be overcome and testing will become an important tool in psychiatry.
阅读本刊更多论文,请点击这里。
