

Unsymmetrical Polysulfidation via Designed Bilateral Disulfurating Reagents
Biography

上海市绿色化学与化工过程绿色化重点实验室
华东师范大学化学与分子工程学院
Jiahui Xue于2013年在西南大学获得硕士学位, 目前在华东师范大学姜雪峰教授课题组做博士,研究领域为多硫化合物化学。
Abstract
Our research group has long been committed to sulfur chemistry. In particular, polysulfide synthesis attracts our attention due to the potential medicinal and material properties of polysulfides. Thus, we designed and synthesized bilateral disulfurating reagents to investigate better methodologies for polysulfide synthesis (Scheme 1). With the assistance of Science of Synthesis Online, we received a comprehensive source of information with high-quality individual articles, with which we gathered information about the usefulness and plausible routes toward the synthesis and application of bilateral disulfurating reagents.
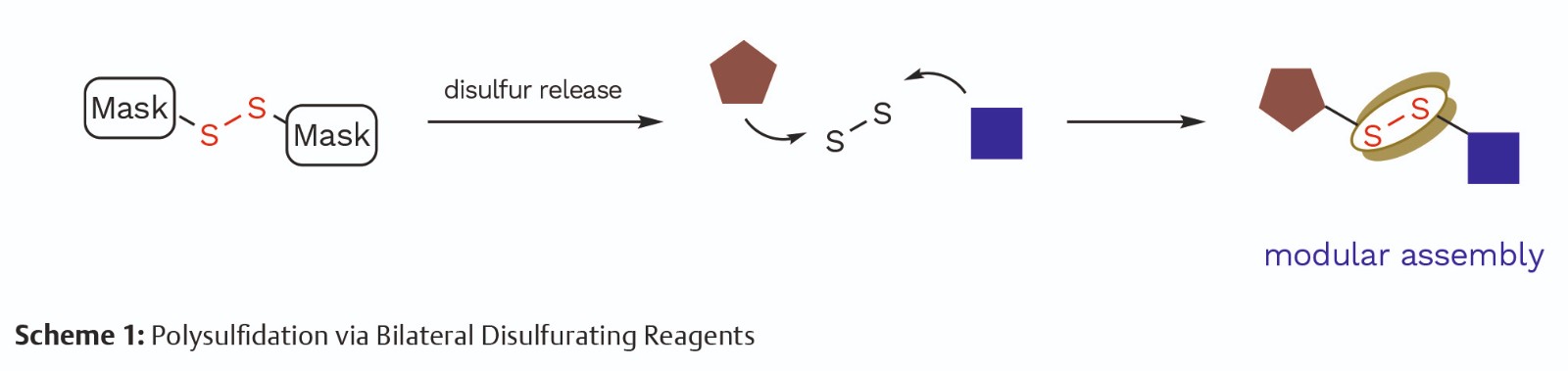
Discussion
Sulfur–sulfur bonds widely exist in the fields of biological, pharmaceutical, and materials chemistry. In organisms, the tertiary structures of proteins are fixed and stabilized via the linkage of sulfur–sulfur bridges between the secondary structures. As a powerful linker, cyclized peptide drugs with sulfur–sulfur bridges have higher stability, activity, and potency compared to the corresponding linear ones. Given the excellent metabolism of the sulfur–sulfur bond in organisms, cutting-edge drug design strategies of antibody-drug conjugates (ADCs) and small molecule drug conjugates (SMDCs) involved disulfur extensively. Polysulfides also possess high-capacity potential in cathode materials for rechargeable lithium batteries.
Science of Synthesis Online is a comprehensive and reliable database, which supplies validated synthetic routines with detailed experimental conditions and procedures in the field of synthetic chemistry. With the assistance of Science of Synthesis Online, we gathered information on dialkoxydisulfide and diaminodisulfide from early research. Based on our concept using the mask effect, we envision that disulfurating reagents with bilateral masks will cross-link two designated functional molecules with sulfur–sulfur bonds sequentially and modularly. After calculating the ring-opening strain, we designed and synthesized a series of cyclic bilateral disulfurating reagents to achieve sequential assembly and modular installation of polysulfides (Scheme 2).
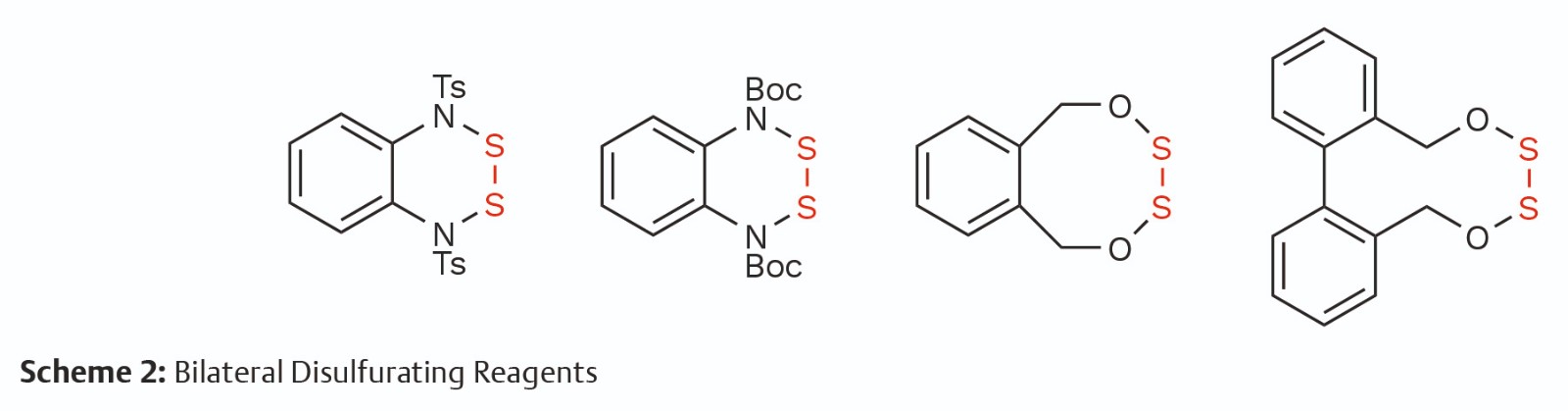
Based on S—O bond dissociation energy nuance, mesocyclic bilateral disulfurating reagents were designed for constructing six species of unsymmetrical polysulfide. Disulfides, trisulfides, and tetrasulfides can be conveniently prepared with amines, mercaptans, arylboronic acids, and electron-rich aromatic molecules. A considerable range of significant life-related molecules could be cross-linked at will with a disulfur bridge to form a variety of diverse functional molecules, which showcases the great potential for application of SMDCs and ADCs. Readily available linear peptide precursors can be tied up using the disulfur fragment to form unique cyclic peptides with a tetraheteroatomic motif (Scheme 3).
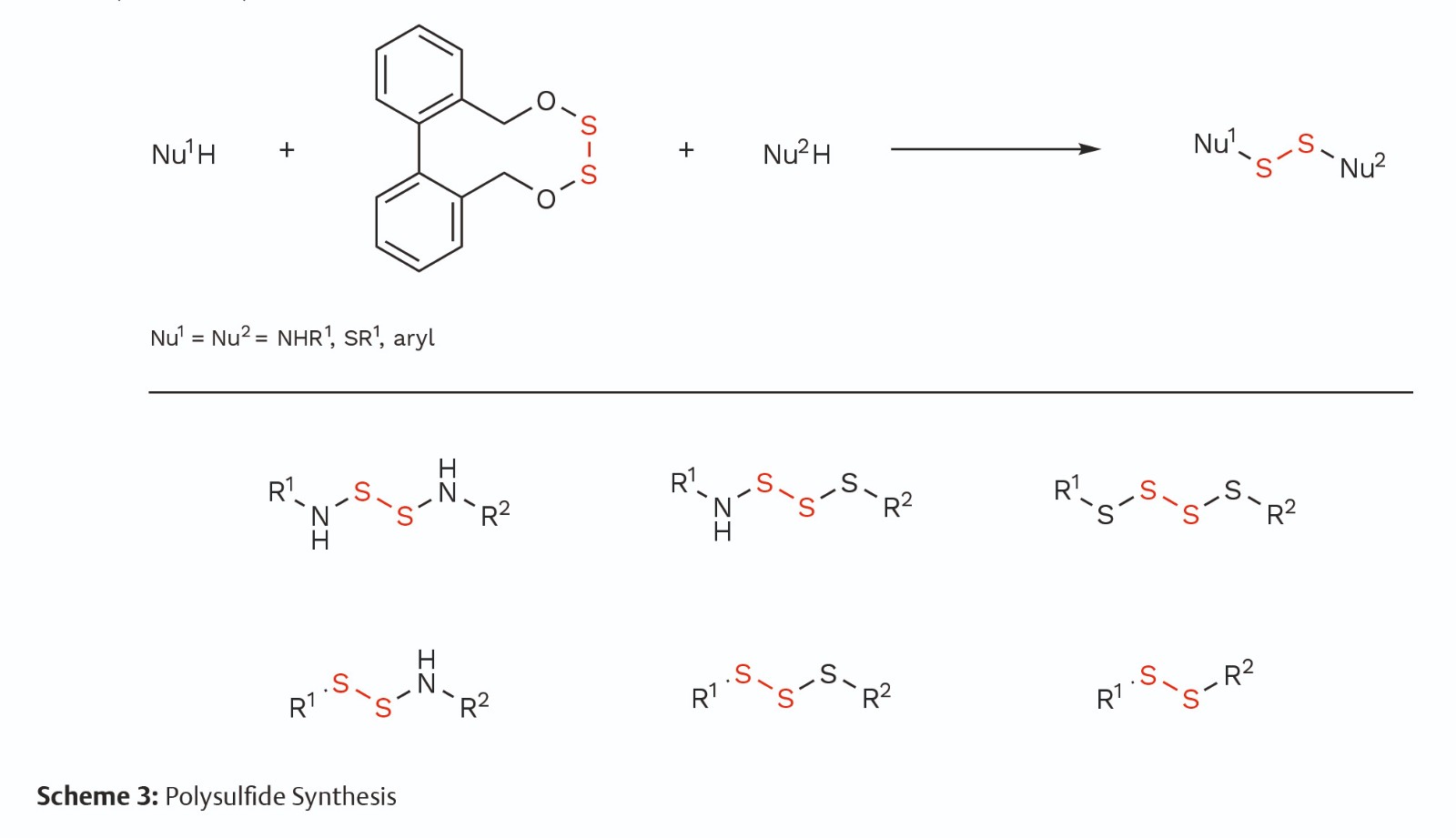
Conclusion
Science of Synthesis Online is a powerful and convenient tool for organic chemists. The internet serves everyone with an ocean of information, from which Science of Synthesis Online could provide an overview of a topic and accurate and reliable experimental conditions and procedures.
14天免费试用:www.thieme.com/sos
