
Thieme有机化学最新一期的精选内容来了!
欢迎在Sustainability & Circularity NOW 上,探索微拟球藻(Nannochloropsis gaditana)如何推动生物源氧化锌合成,以及SSbD框架如何助力材料科学领域更安全、更可持续的创新实践。
在SYNTHESIS与SYNLETT上探索前沿研究:从二苯并噻吩盐的合成到硫醚构建新策略,从迭代同系化反应到螺环丙烷骨架构建。SynOpen 还重点介绍了通过VNS进行卟啉功能化 — 一种创建复杂结构的强大方法。
敬请畅享本期精选内容!
Sustainability & Circularity NOW
Highlight Articles
Unveiling the Role of the Microalga Nannochloropsis gaditana in the Biogenic Synthesis of Zinc Oxide
Tinello Susanna et al.
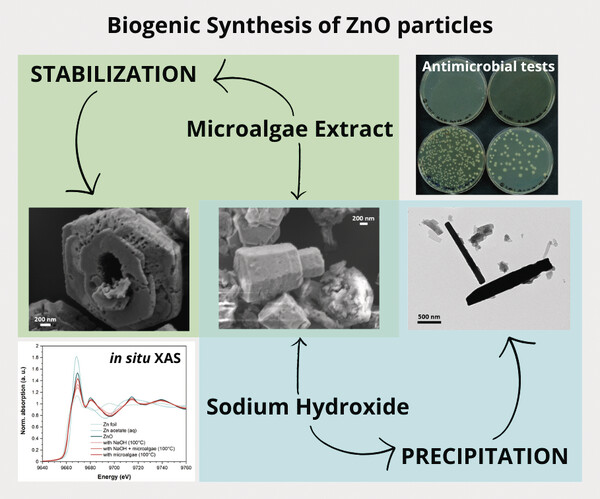
In this article, Gross et al. explore a green, one-pot synthesis of zinc oxide nanoparticles using extracts from the microalga Nannochloropsis gaditana, demonstrating its potential as a sustainable biogenic platform.
Garmendia Aguirre Irantzu et al.
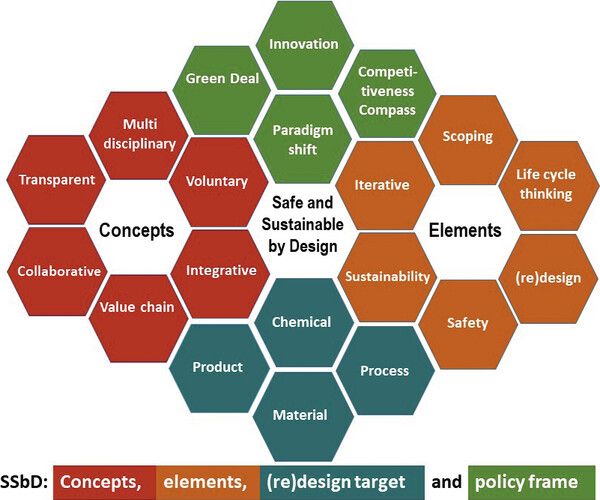
In this short review, Garmendia Aguirre et al. introduce the EU's Safe and Sustainable by Design (SSbD) framework – a new approach to guide safer, more sustainable innovation in chemicals and materials from the design stage onward.
Synlett
Highlight Articles
Synthesis of Dibenzothiophenium Salts and Observations in Radiofluorination
Juntian Zhang, Wei Zhang, Adam T. Hoye, Nathaniel C. Lim, Hui Xiong
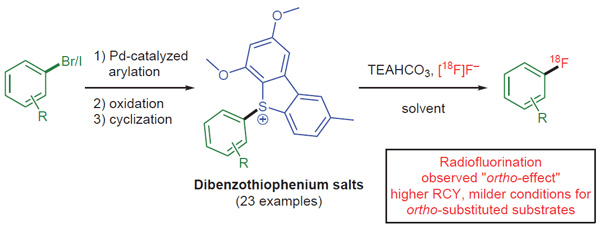
Check out the new synthesis of dibenzothiophenium salts by Juntian Zhang, Wei Zhang, and colleagues, featuring a straightforward three-step process.
One Carbon at a Time: Unlocking Iterative Carboxylic Acid Homologation
Emilie Wheatley, Mattia Silvi
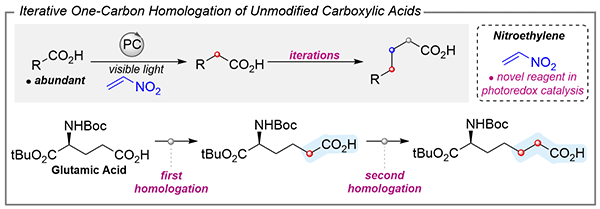
Discover Emilie Wheatley and Mattia Silvi’s innovative approach to iterative carboxylic acid homologation using visible-light photoredox catalysis.
The Duality of Dioxolanyl Radicals towards C–C Bond Construction
K. L. Samony et al.
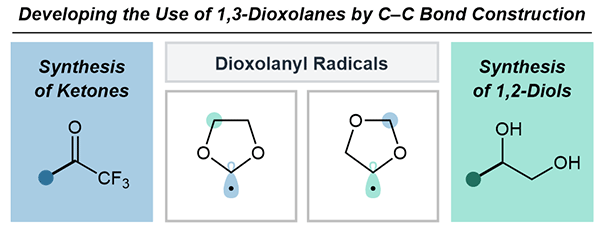
Dive into the latest work by Kim et al. on dioxolanyl radicals for C–C bond formation. The article shows how these radicals yield trifluoromethyl ketones and 1,2-diols.
SynOpen
Highlight Article
Vicarious Nucleophilic Substitution of Hydrogen: An Excellent Tool for Porphyrin Functionalization
S. Ostrowski, A. Mikus
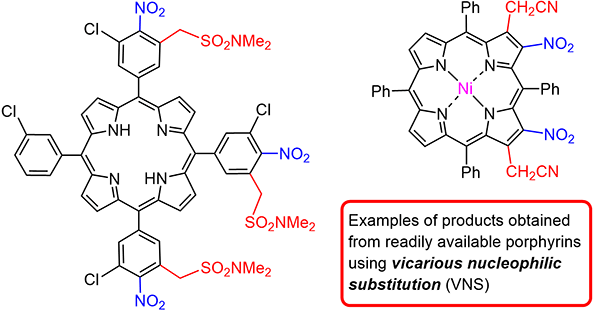
Uncover how Ostrowski and Mikus harness vicarious nucleophilic substitution (VNS) as a powerful strategy for porphyrin functionalization. This graphical review showcases how VNS enables the introduction of multiple substituents, paving the way for complex porphyrin-based architectures.
Synthesis
Highlight Articles in Issue 13
Photocatalytic Strategies for the Synthesis of Xanthates and Their Analogues
L. Geniller et al.
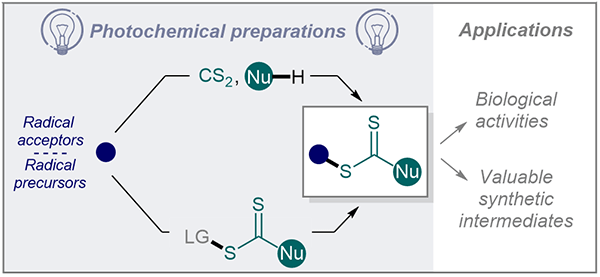
Explore recent developments in photocatalytic methods for synthesizing xanthates and dithiocarbamates in this Short Review by Geniller et al., featuring multi-component and direct functionalization strategies.
Recent Advances in Deoxygenative Thioether Synthesis Using Oxygenated Sulfur Surrogates
L. Y. Lam, C. Ma
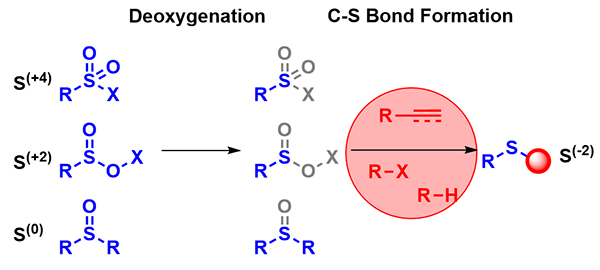
Discover innovative strategies in thioether synthesis without thiols in this recent review by Lam and Ma, highlighting oxygenated sulfur surrogates and key reaction types like cross-coupling and C–H functionalization.
Highlight Articles in Issue 14
C. D. Patel et al.
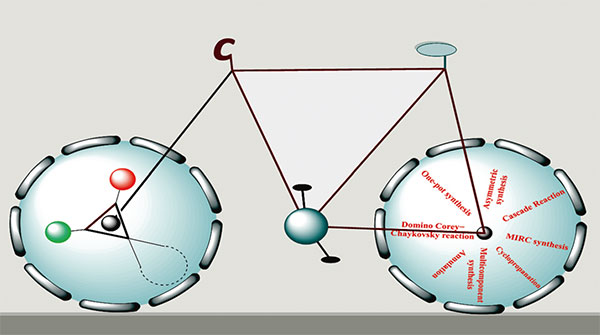
Check out synthetic strategies to access spirocyclopropane scaffolds in this comprehensive review by Patel et al., covering key methodologies from cascade reactions to MIRC and multicomponent approaches centered on the oxindole core.
A. E. Ion et al.
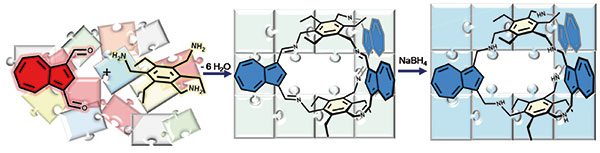
Introducing a novel fluorescent [2+3] imine cage constructed from azulene: Ion et al. present a structurally confirmed system – via NMR and X-ray diffraction – that exhibits distinct redox behavior and solvent-sensitive tunable stability.
