
Process Understanding Enables Control of Hydrogen Fluoride Formation in a Nitroarene Reduction
Contributors:Antonia F. Stepan (Roche), Ferdinand H. Lutter (Janssen Pharmaceutica), Nadine Kuhl (MSD)
Significance
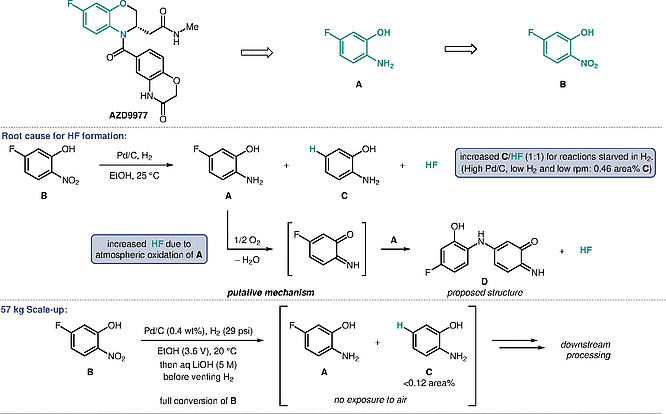
During the development of a synthesis to the nonsteroidal, selective mineralocorticoid receptor (MR) modulator AZD9977, Mallia et al. observed the unexpected formation of defluorinated byproduct C during the nitro reduction of nitrophenol B to aniline intermediate A. The formation of C was found to correlate with the formation of hydrogen fluoride (HF) which posed an environmental and safety hazard. The authors optimized the process to suppress HF formation during the reaction and implemented a basic reaction to sequester any residual HF generated which resulted in a scalable process to aniline intermediate A.
Comment
To minimize HF formation in the nitro reduction of B, the impact of all reaction parameters on the formation of C and by extension HF was studied. It was shown that reactions starved in H2, i.e., reactions with high Pd/C loadings, low H2 pressure and low agitation (rpm), resulted in high levels of C. Severe etching of a glass coupon could be observed when a reaction solution of A was stored under air. This further suggested that oxidative decomposition of A likely proceeding via intermediate D could also release HF. For a 57 kg scale-up, reaction conditions minimizing the formation of C were selected and a LiOH quench was implemented to quench any HF generated such that the reaction stream of A after Pd/C removal could be used in the downstream chemistry without further purification.
Mallia CJ. et al. AstraZeneca, Macclesfield, UK
Control of Hydrogen Fluoride Formation and Release during a Large-Scale Nitroarene Reduction in the Manufacture of AZD9977
Org. Process Res. Dev. 2023;
27: 167-178, DOI: 10.1021/acs.oprd.2c00323.
