
Harnessing Biocatalytic Cascades to Access Pharmaceutically Relevant Piperidines
Contributors: Antonia F. Stepan (Roche), Nadine Kuhl (MSD)
Significance
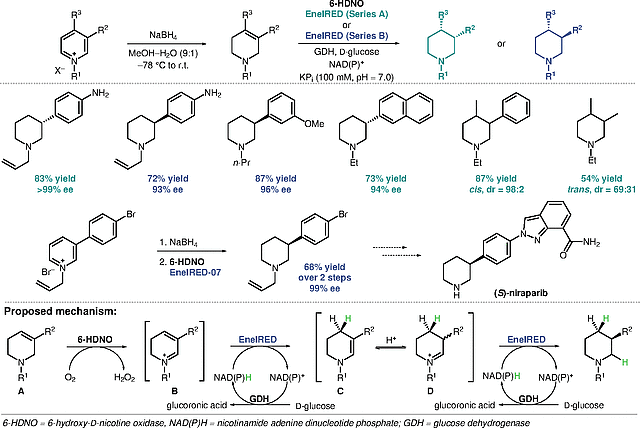
Saturated heterocycles such as piperidines are prevalent structural motifs in pharmaceuticals. However, the synthesis of chiral piperidines with various substitution patterns remains challenging, especially the access to 3- and 3,4-disubstituted derivatives. Turner et al. developed a chemo-enzymatic approach to synthesize 3-substituted piperidines via the dearomatization of pyridinium salts. The new methodology enables the synthesis of pharmaceutically relevant building blocks in high enantioselectivity, such as a 3-arylpiperidine en route to PARP-inhibitor niraparib.
Comment
The key step of the piperidine synthesis by Turner et al. is an amine oxidase/ene-imine reductase (EneIRED) biocatalytic cascade. The first step of the cascade involves the oxidation of the chemically generated tetrahydropyridine A using amine oxidase 6-HDNO to generate pyridinium ion B. The latter undergoes reduction to enamine intermediate C via an EneIRED-mediated conjugate addition of a hydride. Enamine C is in equilibrium with iminium intermediate D from which an EneIRED-mediated reduction to the desired piperidine enantiomer via dynamic kinetic resolution takes place. Screening of various EneIRED panels resulted in the identification of complementary EneIRED series A and B which allow the synthesis of either enantiomer of a desired substrate. This article has also been highlighted with a different focus in the section "Organo- and Biocatalysis" of this issue: Synfacts 2023, 19, 293.
Harawa V, Thorpe TW, Marshall JR, Sangster JJ, Gilio AK, Pirvu L, Heath RS, Angelastro A, Finnigan JD, Charnock SJ, Nafie JW, Grogan G, Whitehead RC, Turner NJ. * University of Manchester, UK
Synthesis of Stereoenriched Piperidines via Chemo-Enzymatic Dearomatization of Activated Pyridines
J. Am. Chem. Soc. 2022; 144: 21088-21095
DOI: 10.1021/jacs.2c07143.
