Total Synthesis of Swinhoeisterol A, Dankasterones A and B, and Periconiastone A
Contributors: Erick M. Carreira, Felix Pultar
Duecker FL, Heinze RC, Heretsch P. * Freie Universität Berlin, Germany
J. Am. Chem. Soc. 2020;
142: 104-108
Key Words
swinhoeisterol A - dankasterone A - dankasterone B - periconiastone A - radical rearrangement - skeleton rearrangement
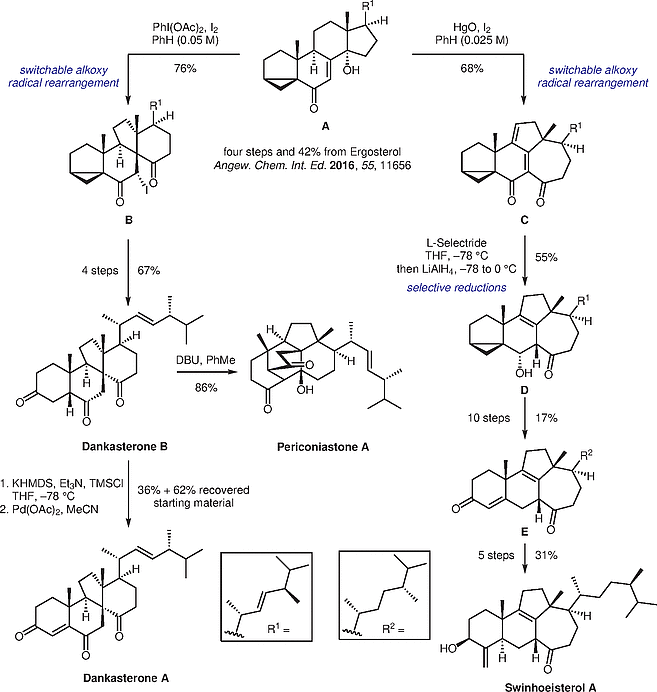
Significance
Heretsch and co-workers report the total synthesis of a number of structurally intriguing natural products from a common intermediate. The concise synthesis is enabled by the strategic application of a switchable alkoxy radical rearrangement.
Comment
Ergosterol is transformed by a known route to cyclopropane A. Two different conditions were developed to lead selectively to B or C. Those advanced intermediates could subsequently be converted into four different complex natural products.
SYNFACTS
阅读SYNFACTS更多论文,请点击 https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0039-1691678。